

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM581978 (DNPSLSIDLTFHLLRTLLELARTQSQRERAEQNRIIFDSV-NH2 (hUcn...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2023285347 | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Receptor SPA binding assays were performed in white 96-well plates in a total volume of 200 mI per well. Freeze dried analogues were dissolved in 80%... | Citation and Details BindingDB Entry DOI: 10.7270/Q23B63Z2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50300149 ((R)-5-Chloro-1-(1-cyclopropylpropyl)-3-(6-(difluor...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-o-CRF from CRF1 receptor in rat frontal cortex homogenate by binding titration assay | J Med Chem 52: 7653-68 (2009) Article DOI: 10.1021/jm900716v BindingDB Entry DOI: 10.7270/Q2CN740W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50026981 (CHEMBL2370939) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Salk Institute Curated by ChEMBL | Assay Description Inhibition of recombinant corticotropin releasing factor receptor 1 assayed using nonselective [125I]-labeled agonist [Tyr0,Glu1,Nle17]-sauvagine | J Med Chem 45: 4737-47 (2002) BindingDB Entry DOI: 10.7270/Q2WQ04JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50300145 ((S)-5-Chloro-1-(cyclopropyl-2-methoxyethyl)-3-[6-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-ovine-CRF from CRF1 receptor in rat frontal cortex homogenate by gamma counting | Bioorg Med Chem Lett 20: 1890-4 (2010) Article DOI: 10.1016/j.bmcl.2010.01.129 BindingDB Entry DOI: 10.7270/Q29G5MZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50300145 ((S)-5-Chloro-1-(cyclopropyl-2-methoxyethyl)-3-[6-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-o-CRF from CRF1 receptor in rat frontal cortex homogenate by binding titration assay | J Med Chem 52: 7653-68 (2009) Article DOI: 10.1021/jm900716v BindingDB Entry DOI: 10.7270/Q2CN740W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50293912 ((R)-5-chloro-1-(1-cyclopropyl-2-methoxyethyl)-3-(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]ovine-CRF from CRF1 receptor in rat frontal cortex by rapid filtration technique | J Med Chem 52: 4161-72 (2009) Article DOI: 10.1021/jm900302q BindingDB Entry DOI: 10.7270/Q2WW7JM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50293912 ((R)-5-chloro-1-(1-cyclopropyl-2-methoxyethyl)-3-(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-ovine-CRF from CRF1 receptor in rat frontal cortex homogenate by gamma counting | J Med Chem 52: 4173-91 (2009) Article DOI: 10.1021/jm900301y BindingDB Entry DOI: 10.7270/Q2G44Q9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50293912 ((R)-5-chloro-1-(1-cyclopropyl-2-methoxyethyl)-3-(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Displacement of [125I]-o-CRF from CRFR1 in rat frontal cortex after 2 hrs by gamma counter | Bioorg Med Chem Lett 22: 6651-5 (2012) Article DOI: 10.1016/j.bmcl.2012.08.112 BindingDB Entry DOI: 10.7270/Q20C4WWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50293974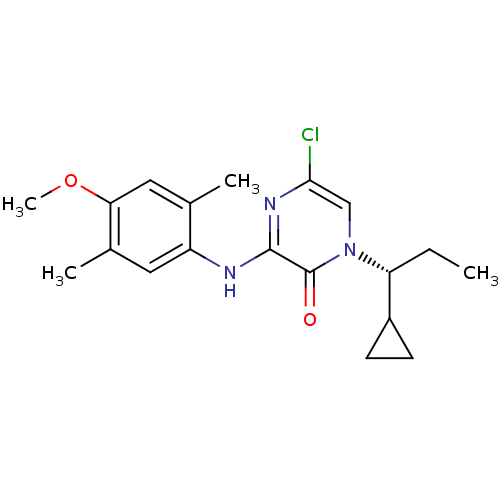 ((R)-5-Chloro-1-(1-cyclopropylpropyl)-3-(4-methoxy-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-ovine-CRF from CRF1 receptor in rat frontal cortex homogenate by gamma counting | J Med Chem 52: 4173-91 (2009) Article DOI: 10.1021/jm900301y BindingDB Entry DOI: 10.7270/Q2G44Q9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50300136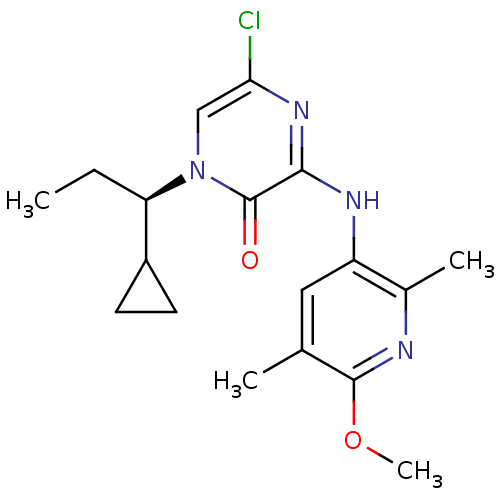 ((R)-5-Chloro-1-(1-cyclopropylpropyl)-3-(6-methoxy-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-o-CRF from CRF1 receptor in rat frontal cortex homogenate by binding titration assay | J Med Chem 52: 7653-68 (2009) Article DOI: 10.1021/jm900716v BindingDB Entry DOI: 10.7270/Q2CN740W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50300145 ((S)-5-Chloro-1-(cyclopropyl-2-methoxyethyl)-3-[6-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-ovine-CRF from CRF1 receptor in rat frontal cortex homogenate after 2 hrs by gamma counting analysis | Bioorg Med Chem Lett 26: 2184-7 (2016) Article DOI: 10.1016/j.bmcl.2016.03.067 BindingDB Entry DOI: 10.7270/Q2KK9FVR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50293964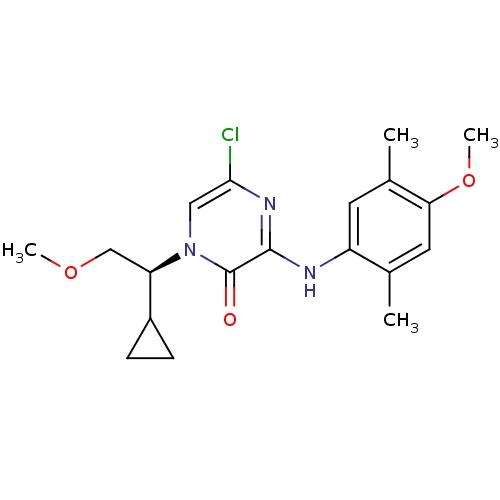 ((S)-5-Chloro-1-(1-cyclopropyl-2-methoxyethyl)-3-(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-ovine-CRF from CRF1 receptor in rat frontal cortex homogenate by gamma counting | J Med Chem 52: 4173-91 (2009) Article DOI: 10.1021/jm900301y BindingDB Entry DOI: 10.7270/Q2G44Q9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50300142 ((R)-5-Chloro-1-(1-cyclopropylethyl)-3-[6-(difluoro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-o-CRF from CRF1 receptor in rat frontal cortex homogenate by binding titration assay | J Med Chem 52: 7653-68 (2009) Article DOI: 10.1021/jm900716v BindingDB Entry DOI: 10.7270/Q2CN740W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50155982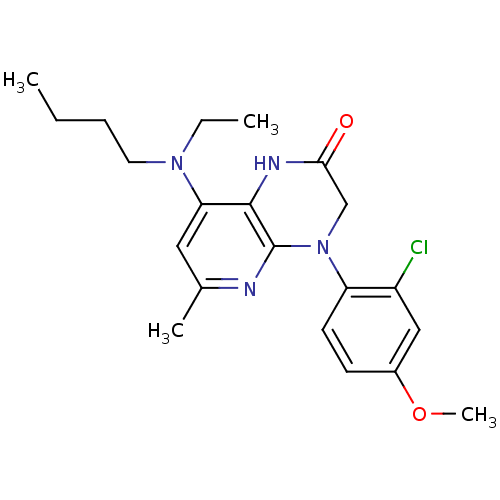 (8-(Butyl-ethyl-amino)-4-(2-chloro-4-methoxy-phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]-ovine-CRF binding to corticotropin releasing factor receptor 1 | J Med Chem 47: 5783-90 (2004) Article DOI: 10.1021/jm049737f BindingDB Entry DOI: 10.7270/Q2S75FT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50300142 ((R)-5-Chloro-1-(1-cyclopropylethyl)-3-[6-(difluoro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-ovine-CRF from CRF1 receptor in rat frontal cortex homogenate by gamma counting | Bioorg Med Chem Lett 20: 1890-4 (2010) Article DOI: 10.1016/j.bmcl.2010.01.129 BindingDB Entry DOI: 10.7270/Q29G5MZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50293941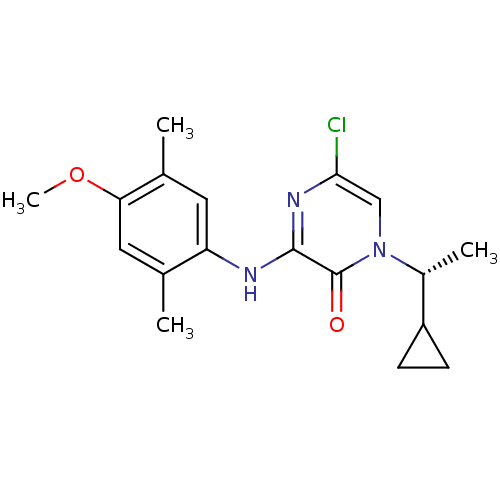 ((R)-5-Chloro-1-(1-cyclopropylethyl)-3-(4-methoxy-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]ovine-CRF from CRF1 receptor in rat frontal cortex by rapid filtration technique | J Med Chem 52: 4161-72 (2009) Article DOI: 10.1021/jm900302q BindingDB Entry DOI: 10.7270/Q2WW7JM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50293941 ((R)-5-Chloro-1-(1-cyclopropylethyl)-3-(4-methoxy-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-ovine-CRF from CRF1 receptor in rat frontal cortex homogenate by gamma counting | J Med Chem 52: 4173-91 (2009) Article DOI: 10.1021/jm900301y BindingDB Entry DOI: 10.7270/Q2G44Q9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50293916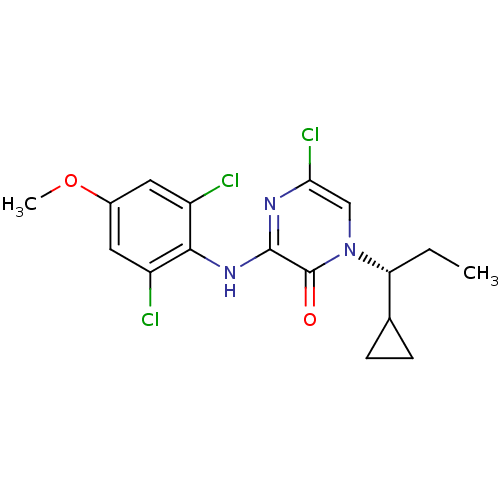 ((R)-5-Chloro-1-(1-cyclopropylpropyl)-3-(2,6-dichlo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]ovine-CRF from CRF1 receptor in rat frontal cortex by rapid filtration technique | J Med Chem 52: 4161-72 (2009) Article DOI: 10.1021/jm900302q BindingDB Entry DOI: 10.7270/Q2WW7JM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50293935 ((R)-5-Chloro-1-(1-cyclopropylethyl)-3-[2,6-dichlor...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]ovine-CRF from CRF1 receptor in rat frontal cortex by rapid filtration technique | J Med Chem 52: 4161-72 (2009) Article DOI: 10.1021/jm900302q BindingDB Entry DOI: 10.7270/Q2WW7JM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50432097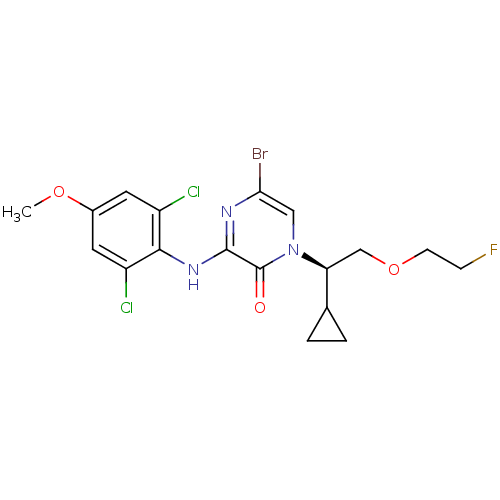 (CHEMBL2349560) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]-o-CRF from CRF1 receptor in rat frontal cortex homogenate after 2 hrs by gamma counting | Bioorg Med Chem Lett 23: 2052-5 (2013) Article DOI: 10.1016/j.bmcl.2013.02.009 BindingDB Entry DOI: 10.7270/Q2DR2WW3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50432099 (CHEMBL2349558) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]-o-CRF from CRF1 receptor in rat frontal cortex homogenate after 2 hrs by gamma counting | Bioorg Med Chem Lett 23: 2052-5 (2013) Article DOI: 10.1016/j.bmcl.2013.02.009 BindingDB Entry DOI: 10.7270/Q2DR2WW3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50155974 (8-(Butyl-ethyl-amino)-4-(2,4-dichloro-phenyl)-6-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]-ovine-CRF binding to corticotropin releasing factor receptor 1 | J Med Chem 47: 5783-90 (2004) Article DOI: 10.1021/jm049737f BindingDB Entry DOI: 10.7270/Q2S75FT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50300144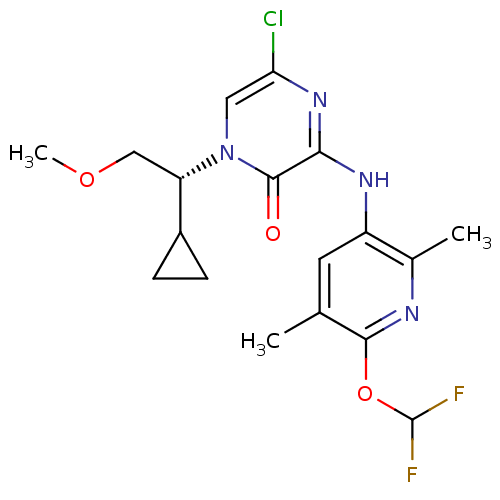 ((R)-5-Chloro-1-(1-cyclopropyl-2-methoxyethyl)-3-[6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-o-CRF from CRF1 receptor in rat frontal cortex homogenate by binding titration assay | J Med Chem 52: 7653-68 (2009) Article DOI: 10.1021/jm900716v BindingDB Entry DOI: 10.7270/Q2CN740W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50155964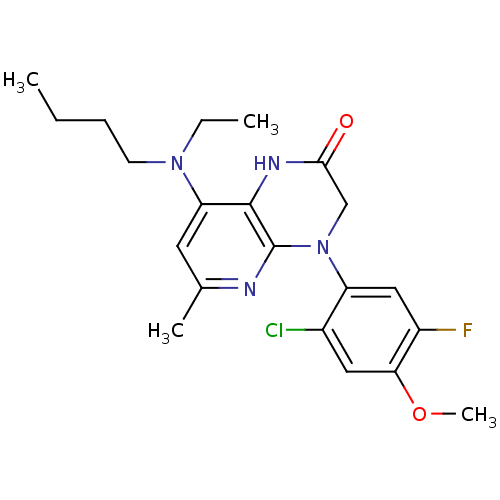 (8-(Butyl-ethyl-amino)-4-(2-chloro-5-fluoro-4-metho...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]-ovine-CRF binding to corticotropin releasing factor receptor 1 | J Med Chem 47: 5783-90 (2004) Article DOI: 10.1021/jm049737f BindingDB Entry DOI: 10.7270/Q2S75FT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50293973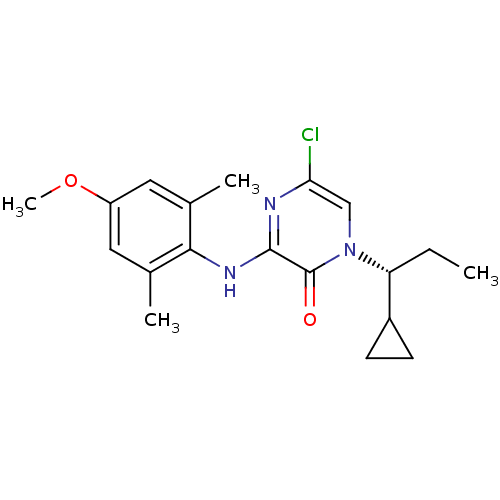 ((R)-5-Chloro-1-(1-cyclopropylpropyl)-3-(4-methoxy-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-ovine-CRF from CRF1 receptor in rat frontal cortex homogenate by gamma counting | J Med Chem 52: 4173-91 (2009) Article DOI: 10.1021/jm900301y BindingDB Entry DOI: 10.7270/Q2G44Q9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50293914 ((R)-5-chloro-1-(1-cyclopropylpropyl)-3-(2,4-dichlo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]ovine-CRF from CRF1 receptor in rat frontal cortex by rapid filtration technique | J Med Chem 52: 4161-72 (2009) Article DOI: 10.1021/jm900302q BindingDB Entry DOI: 10.7270/Q2WW7JM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50293914 ((R)-5-chloro-1-(1-cyclopropylpropyl)-3-(2,4-dichlo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-ovine-CRF from CRF1 receptor in rat frontal cortex homogenate by gamma counting | J Med Chem 52: 4173-91 (2009) Article DOI: 10.1021/jm900301y BindingDB Entry DOI: 10.7270/Q2G44Q9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50293953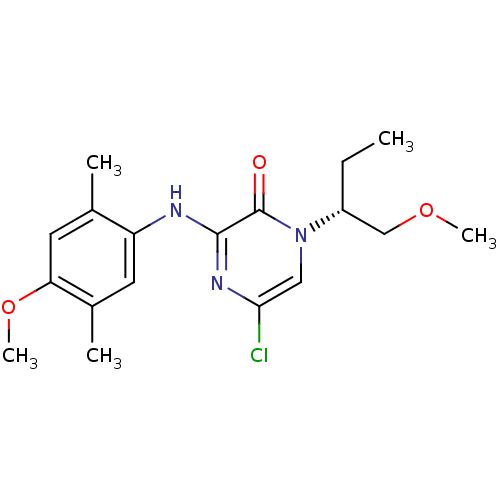 ((R)-5-Chloro-3-(4-methoxy-2,5-dimethylphenylamino)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-ovine-CRF from CRF1 receptor in rat frontal cortex homogenate by gamma counting | J Med Chem 52: 4173-91 (2009) Article DOI: 10.1021/jm900301y BindingDB Entry DOI: 10.7270/Q2G44Q9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50432098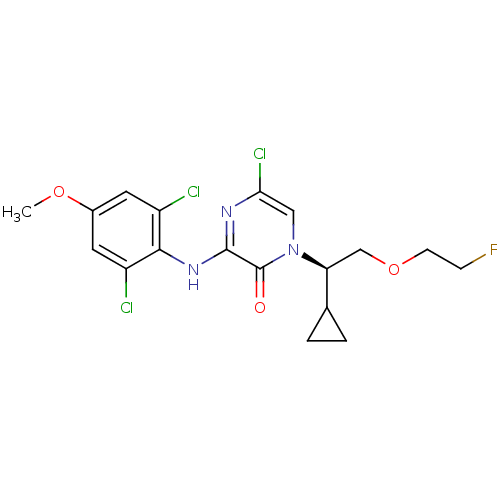 (CHEMBL2349559) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]-o-CRF from CRF1 receptor in rat frontal cortex homogenate after 2 hrs by gamma counting | Bioorg Med Chem Lett 23: 2052-5 (2013) Article DOI: 10.1016/j.bmcl.2013.02.009 BindingDB Entry DOI: 10.7270/Q2DR2WW3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50432102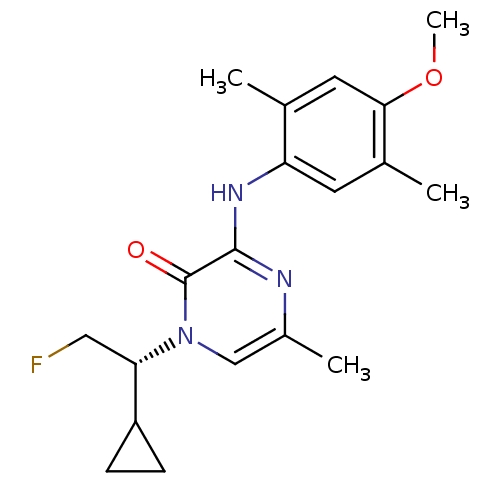 (CHEMBL2349570) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]-o-CRF from CRF1 receptor in rat frontal cortex homogenate after 2 hrs by gamma counting | Bioorg Med Chem Lett 23: 2052-5 (2013) Article DOI: 10.1016/j.bmcl.2013.02.009 BindingDB Entry DOI: 10.7270/Q2DR2WW3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50293981 ((R)-5-Chloro-3-(7-chloro-5-methoxyindolin-1-yl)-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-ovine-CRF from CRF1 receptor in rat frontal cortex homogenate by gamma counting | J Med Chem 52: 4173-91 (2009) Article DOI: 10.1021/jm900301y BindingDB Entry DOI: 10.7270/Q2G44Q9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50293952 (5-Chloro-3-(4-methoxy-2,5-dimethylphenylamino)-1-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-ovine-CRF from CRF1 receptor in rat frontal cortex homogenate by gamma counting | J Med Chem 52: 4173-91 (2009) Article DOI: 10.1021/jm900301y BindingDB Entry DOI: 10.7270/Q2G44Q9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50293913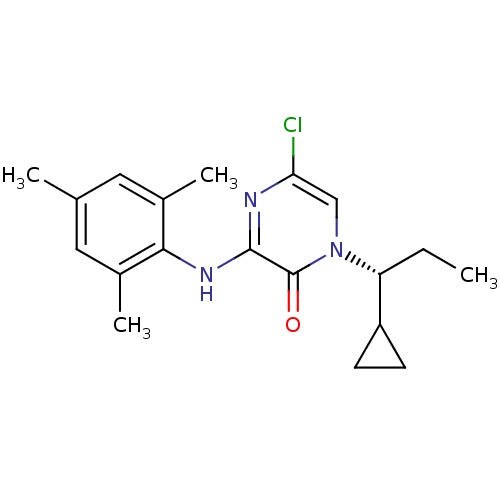 ((R)-5-Chloro-1-(1-cyclopropylpropyl)-3-(2,4,6-trim...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]ovine-CRF from CRF1 receptor in rat frontal cortex by rapid filtration technique | J Med Chem 52: 4161-72 (2009) Article DOI: 10.1021/jm900302q BindingDB Entry DOI: 10.7270/Q2WW7JM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50293913 ((R)-5-Chloro-1-(1-cyclopropylpropyl)-3-(2,4,6-trim...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-ovine-CRF from CRF1 receptor in rat frontal cortex homogenate by gamma counting | J Med Chem 52: 4173-91 (2009) Article DOI: 10.1021/jm900301y BindingDB Entry DOI: 10.7270/Q2G44Q9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50313860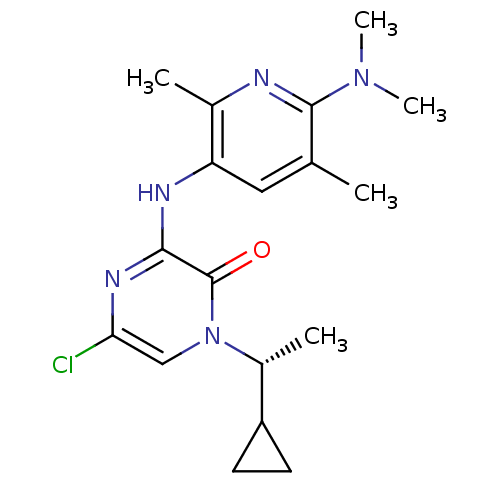 ((R)-5-chloro-1-(1-cyclopropylethyl)-3-(6-(dimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-ovine-CRF from CRF1 receptor in rat frontal cortex homogenate by gamma counting | Bioorg Med Chem Lett 20: 1890-4 (2010) Article DOI: 10.1016/j.bmcl.2010.01.129 BindingDB Entry DOI: 10.7270/Q29G5MZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50387635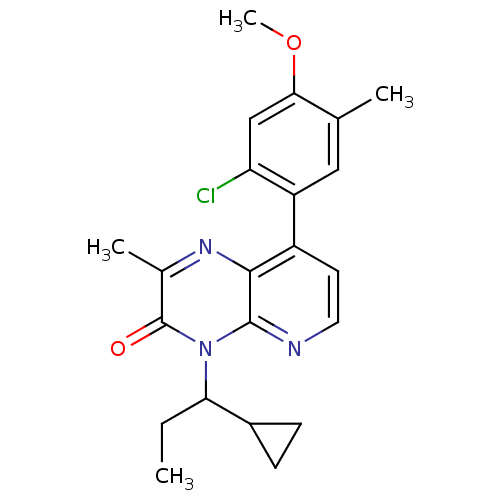 (CHEMBL2058338) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co Curated by ChEMBL | Assay Description Antagonist activity at rat CFR1R | Bioorg Med Chem Lett 22: 4986-9 (2012) Article DOI: 10.1016/j.bmcl.2012.06.034 BindingDB Entry DOI: 10.7270/Q23X87Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50395826 (CHEMBL2165202) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Displacement of [125I]-o-CRF from CRFR1 in rat frontal cortex after 2 hrs by gamma counter | Bioorg Med Chem Lett 22: 6651-5 (2012) Article DOI: 10.1016/j.bmcl.2012.08.112 BindingDB Entry DOI: 10.7270/Q20C4WWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50155967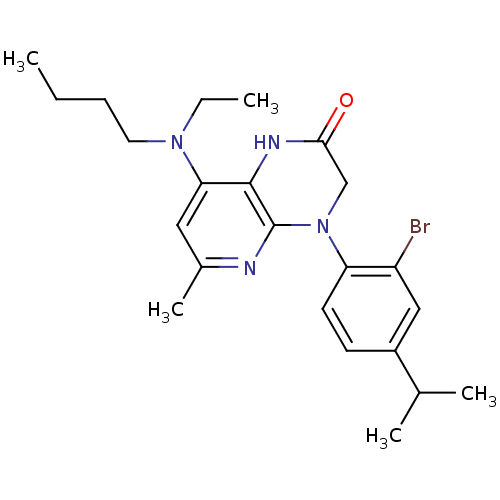 (4-(2-Bromo-4-isopropyl-phenyl)-8-(butyl-ethyl-amin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]-ovine-CRF binding to corticotropin releasing factor receptor 1 | J Med Chem 47: 5783-90 (2004) Article DOI: 10.1021/jm049737f BindingDB Entry DOI: 10.7270/Q2S75FT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50370170 (CHEMBL1794009) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a |
Salk Institute Curated by ChEMBL | Assay Description Inhibition of recombinant corticotropin releasing factor receptor 1 assayed using nonselective [125I]-labeled agonist [Tyr0,Glu1,Nle17]-sauvagine | J Med Chem 45: 4737-47 (2002) BindingDB Entry DOI: 10.7270/Q2WQ04JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50501032 (CHEMBL3799870) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-ovine-CRF from CRF1 receptor in rat frontal cortex homogenate after 2 hrs by gamma counting analysis | Bioorg Med Chem Lett 26: 2184-7 (2016) Article DOI: 10.1016/j.bmcl.2016.03.067 BindingDB Entry DOI: 10.7270/Q2KK9FVR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50293951 (5-Chloro-1-(1-ethylpropyl)-3-(4-methoxy-2,5-dimeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-ovine-CRF from CRF1 receptor in rat frontal cortex homogenate by gamma counting | J Med Chem 52: 4173-91 (2009) Article DOI: 10.1021/jm900301y BindingDB Entry DOI: 10.7270/Q2G44Q9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50313869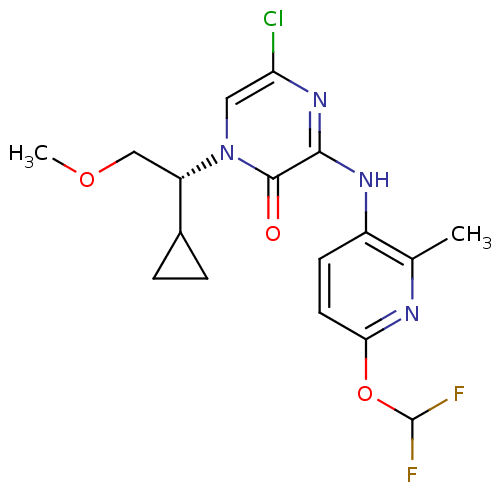 ((R)-5-chloro-1-(1-cyclopropyl-2-methoxyethyl)-3-(6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-ovine-CRF from CRF1 receptor in rat frontal cortex homogenate by gamma counting | Bioorg Med Chem Lett 20: 1890-4 (2010) Article DOI: 10.1016/j.bmcl.2010.01.129 BindingDB Entry DOI: 10.7270/Q29G5MZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50300146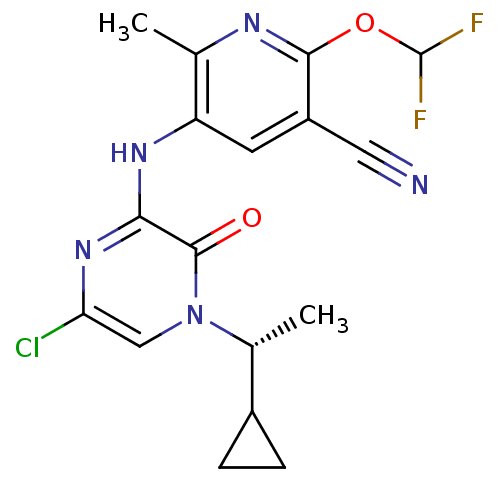 ((R)-4-(1-Cyclopropylethyl)-6-(6-(difluoromethoxy)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-o-CRF from CRF1 receptor in rat frontal cortex homogenate by binding titration assay | J Med Chem 52: 7653-68 (2009) Article DOI: 10.1021/jm900716v BindingDB Entry DOI: 10.7270/Q2CN740W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM20977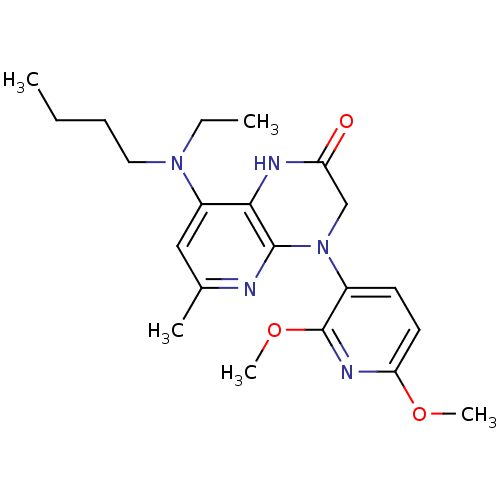 (8-[butyl(ethyl)amino]-4-(2,6-dimethoxypyridin-3-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]-ovine-CRF binding to corticotropin releasing factor receptor 1 | J Med Chem 47: 5783-90 (2004) Article DOI: 10.1021/jm049737f BindingDB Entry DOI: 10.7270/Q2S75FT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM20977 (8-[butyl(ethyl)amino]-4-(2,6-dimethoxypyridin-3-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | 7.0 | 23 |
Bristol-Myers Squibb Company | Assay Description The IC50 values were measured by monitoring the inhibition effects of test compounds on [125I]-o-CRF binding to rat cortex in vitro. | J Med Chem 50: 2269-72 (2007) Article DOI: 10.1021/jm0611410 BindingDB Entry DOI: 10.7270/Q25Q4TCS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50387601 (CHEMBL2058596) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co Curated by ChEMBL | Assay Description Antagonist activity at rat CFR1R | Bioorg Med Chem Lett 22: 4986-9 (2012) Article DOI: 10.1016/j.bmcl.2012.06.034 BindingDB Entry DOI: 10.7270/Q23X87Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50300147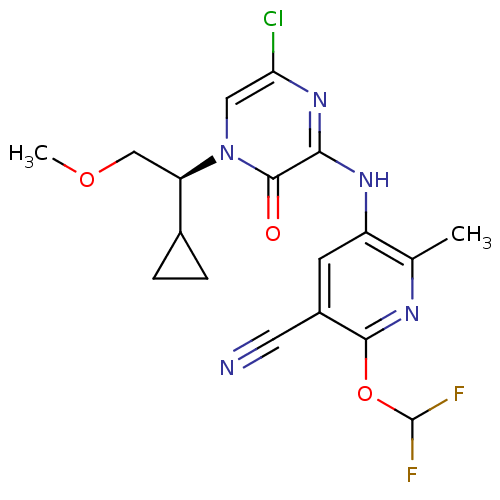 ((S)-4-(1-Cyclopropyl-2-methoxyethyl)-6-[6-(difluor...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.860 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-o-CRF from CRF1 receptor in rat frontal cortex homogenate by binding titration assay | J Med Chem 52: 7653-68 (2009) Article DOI: 10.1021/jm900716v BindingDB Entry DOI: 10.7270/Q2CN740W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50293967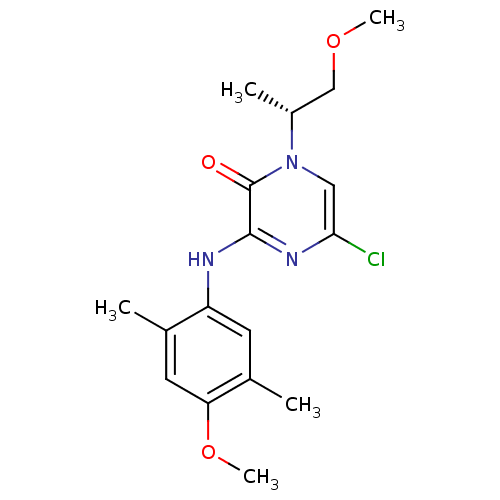 ((R)-5-Chloro-3-(4-methoxy-2,5-dimethylphenylamino)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.880 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Displacement of [125I]-o-CRF from CRFR1 in rat frontal cortex after 2 hrs by gamma counter | Bioorg Med Chem Lett 22: 6651-5 (2012) Article DOI: 10.1016/j.bmcl.2012.08.112 BindingDB Entry DOI: 10.7270/Q20C4WWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50320241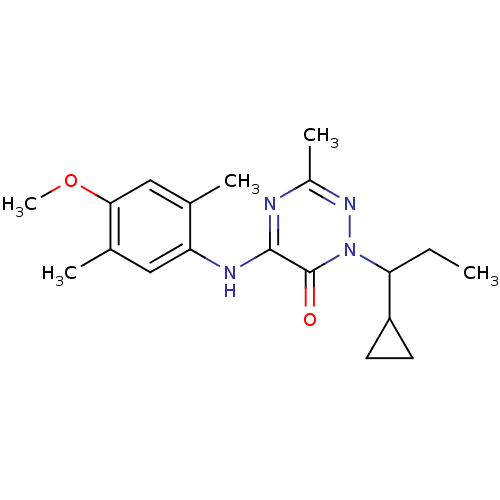 (1-(1-cyclopropylpropyl)-5-(4-methoxy-2,5-dimethylp...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-ovine CRF from rat CRF1 receptor | Bioorg Med Chem Lett 20: 3579-83 (2010) Article DOI: 10.1016/j.bmcl.2010.04.121 BindingDB Entry DOI: 10.7270/Q2SX6DDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50158983 (CHEMBL439883 | E G P P I S I D L S L E L L R K M I...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Salk Institute Curated by ChEMBL | Assay Description Inhibition of recombinant corticotropin releasing factor receptor 1 assayed using nonselective [125I]-labeled agonist [Tyr0,Glu1,Nle17]-sauvagine | J Med Chem 45: 4737-47 (2002) BindingDB Entry DOI: 10.7270/Q2WQ04JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1258 total ) | Next | Last >> |